Current Research
The “elusive”
complexes of heme-modeling iron-porphyrins with nitrogen dioxide (NO2)
have been obtained and spectrally characterized (Scheme 1). It is 5-coordinate
O-bound nitrito complex Fe(Por)(ONO). These species
are fairly stable at ambient conditions and slowly decomposes within few days
resulting mostly iron nitrosyls. This observation throws some light on the
mechanism of vasodilatation activity of nitrite ion in mammalian biology.
Scheme
1.
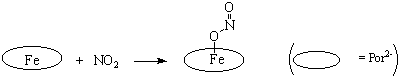
Some chemical
properties of this new complex are studied:
Interaction with
nitric oxide (NO) at low temperature conditions leads to the formation of
nitrito-nitrosyl complex Fe(Por)(NO)(ONO) that
isomerizes to more stable known nitro-nitrosyl species Fe(Por)(NO)(NO2)
upon warming (Scheme 2).
Scheme 2.
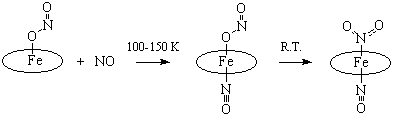
Isomerization occurs
through the mechanism of NO2 flipping from O- to N-bound form rather
than the elimination of O-coordinated NO2 ligand followed by
formation of new Fe-N(NO2) bond.
Interaction with
electron-donor ligands (B) leads to the formation of 6-coordinate nitro complexes
Fe(Por)(NO2)(B).
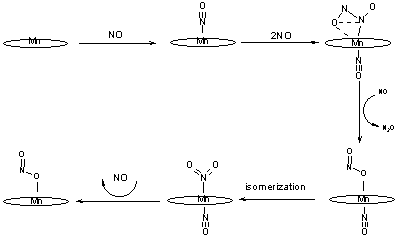
Low-temperature NO disproportionation reaction performed by solid state Mn-Porphyrins has been discovered and studied in detail. The mechanism of disproportionation reaction is offered according to the scheme 3. Incidentally new 6-coordinate nitrito-nitrosyl and nitro-nitrosyl complexes {Mn(Por)(ONO)(NO) and Mn(Por)(NO2)(NO)} were identified at low-temperature conditions.
Scheme
3.
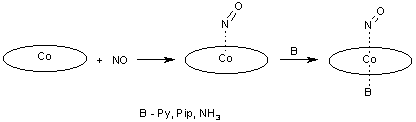
Previously unknown 6-coordinate nitrosyl complexes of Co-porphyrins with trans N-donor ligands have been obtained at low-temperature conditions and spectrally characterized. (Scheme 4).
Scheme
4.
The same behavior
reveals Co-complexes of mono-4(3)-pyridyl-triphenyl porphyrins that in sublimed
layers form coordination oligomers. Low-temperature interaction of NO gas with
these self-organized supramolecular assemblies leads to the formation of 5- and
6-coordinate nitrosyl complexes represented at the Fig.

These microporous
systems containing unsaturated metal atoms in their frameworks reveal promising
properties to serve as a solid state NO-storage agents and selective
adsorbents.